Working With the FDA on Genetic Health Risk Reports
23andme
Background
I played a lead role in enabling 23andMe to provide health reports based on genetic screening. This was a groundbreaking (and thoroughly designed) process that satisfied the FDA.
In February 2015, the FDA cleared the first ever consumer-facing genetic health report to be reviewed by the FDA. This enabled 23andMe to provide genetic health reports to consumers. We demonstrated, to the FDA, that over 90% of users in our user comprehension study could accurately answer what the test is /is not for, what the result is, and what the results mean.
After the FDA’s first approval of Carrier Status reports, 23andMe made a second class II submission in 2015 called Genetic Health Risk reports. These reports communicate an individual’s predisposition, or personal health risk, for developing a certain health condition. This report category covers more sensitive health conditions, such as Alzheimer’s and Parkinson’s diseases, as well as lower risk reports, such as Hereditary Thrombophilia, G6PD Deficiency, AATD. I worked end-to-end in this process, from initial concepts to prototypes to the user comprehension testing platform to the final FDA-cleared designs.
"Finally, the company conducted a user study comprised of 667 randomly recruited participant representing the U.S general population in age, gender, race and education level to show the test instructions and results were easy to follow and understand."
The New Challenge
The Genetic Health risk reports are complex. In order to pass FDA approval, the FDA had to run an independent study to determine if users are able to comprehend the report, without the assistance of a scientist. 90% of individuals need to pass this independent study. This comprehension is important, because the reports may contain sensitive information that may trigger various emotional states, within the users. The user needs to understand the scientific data.
User Testing
Between February 2015 and March 2016, over 8500 people were shown the Genetic Health Risk reports. We ran 14 different qualitative and quantitative studies, using various forms of prototypes on mobile and desk top applications.
We designed and built our own testing platform, for desktop and mobile web, to analyze and understand all the various reasons why a user would not comprehend any section of the report. For each study, we rapidly incorporated feedback and iterated on the existing designs, in preparation for clinical study. In addition to iterating for comprehension, we incorporated key product metrics to assess the satisfaction with product, and probed into the emotional reaction of uses to the types of information reported.
Creating Educational Content For The Report
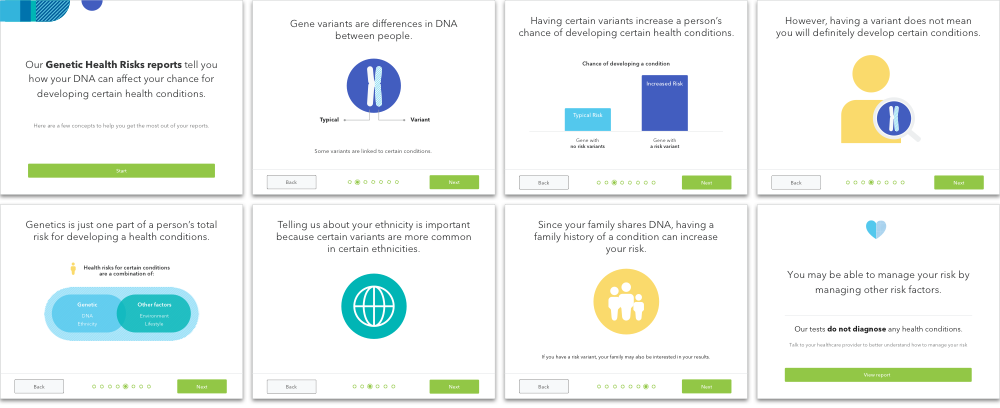
Genetics is complicated. One initial learning was that the report alone may not be sufficient for communicating all the necessary information. Certain concepts were foundational for a user to understand the report, and to accommodate these concepts, we designed these educational slides to prime the user before reviewing his or her result. The educational module is part of user testing as well.
Final Genetic Health Risk Report Designs
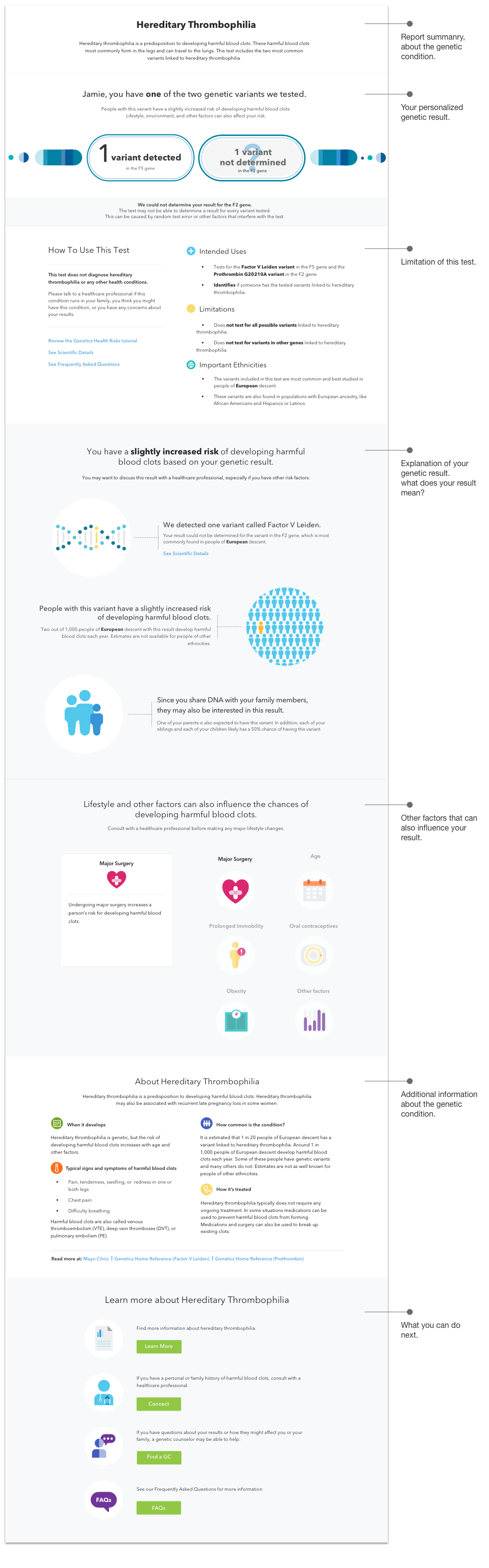
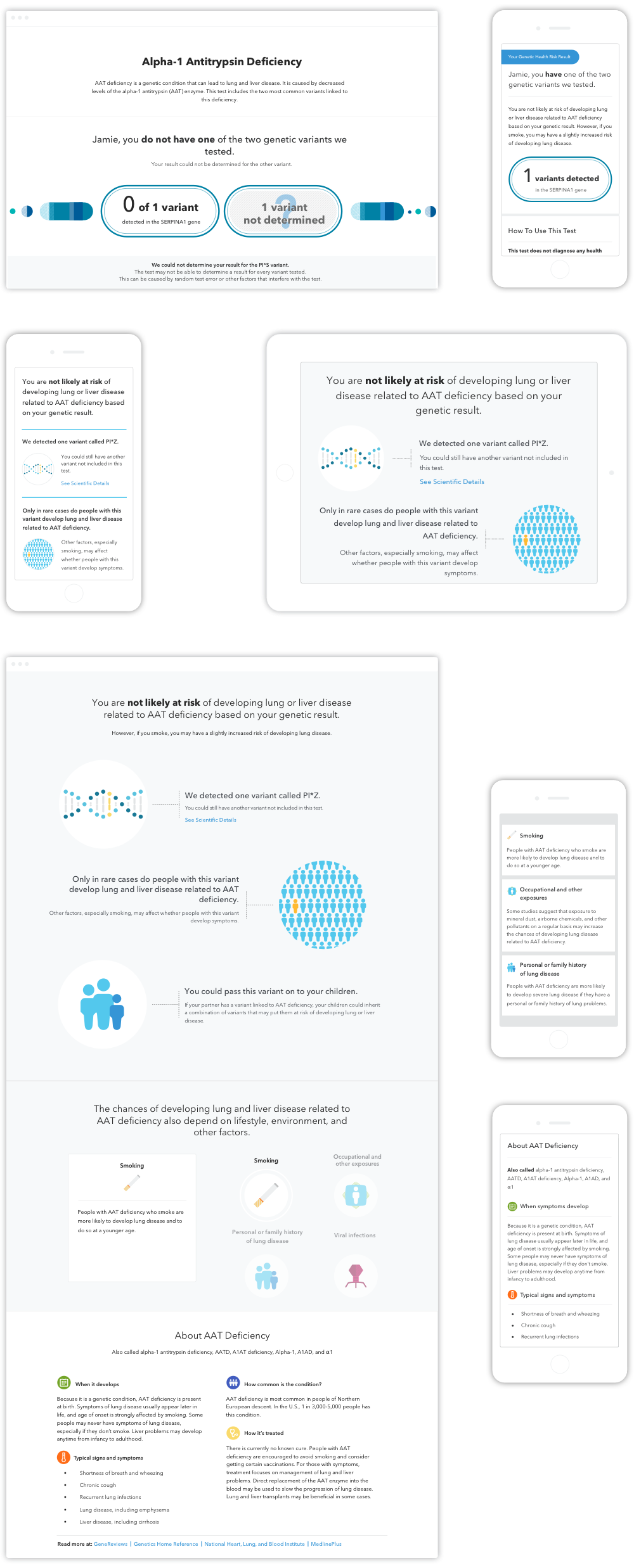
Below shows two Genetic Health Risk reports. Each report has 10 different result outcomes. The report design has to accommodate all result scenarios. Modular design is a collaborative approach to building these reports. Thinking in re-usable components, rather than full page of new layout, helps user to move to the next report quicker and scale the product in a more consistent flexible way.
What's Next?
Once the design and the FDA user studies were complete, I continued to partner with the Scientists to refine the designs. I'm now working with Engineering to develop and implement the reports to future launch. After two years of working on this project, once we receive the clearance, the end product for four Genetic Health Risks reports will be shipping to our customers.